Case Study: Self-Lubricated Polyisoprene for Medical Septum Applications
The project
Develop a system that reduces the needle drag and piercing resistance of the septum and injection site materials to increase product performance.
The solution
Chemists developed a family of self-lubricated polyisoprene materials that have been manufactured with a proprietary lubricant system and show a minimal reduction of physical and mechanical properties.
By Saman Nanayakkara and Shu Peng
Due to its availability as an ISO 10993 medical grade compound, polyisoprene rubber, which has a unique set of combined mechanical and chemical properties, has been widely used in medical device applications. The material is ideal for septums and injection sites for medical fluid transfer applications. Medical grade polyisoprene compounds have high tear strength and high elastic resilience. These characteristics can provide the desired resealability properties of the septum or injection site after piercing one or more times with a needle.
Medical device manufacturers have long sought a reduction in needle drag or piercing resistance of septum and injection site materials to increase product performance. Post molding surface treatment to modify coefficient of friction is the conventional approach taken to reduce tackiness for improved part handling. This process, however, is a surface treatment for reducing surface friction and does not effectively reduce needle drag, which is caused largely by friction within the septum and injection site materials. Furthermore, this secondary surface treatment adds additional cost to the component.
It would, therefore, be ideal if the polyisoprene compound used to mold the septum and injection site could be lubricious as opposed to only a surface-treated component. Chemists have generally borrowed the idea of self-lubricating compounds developed for non-medical applications to add lubricity, in while silicone oil is widely used as the lubricating agent. It has been found that adding silicone oil to rubber compounds can greatly help reduce friction.
However, for medical grade polyisoprene compounds, adding silicone oil can adversely impact the physical and mechanical properties. This negative impact of the silicone fluid on the overall physical properties of the polyisoprene compound tends to make it a less attractive material for septums and injection sites used in drug infusion applications.
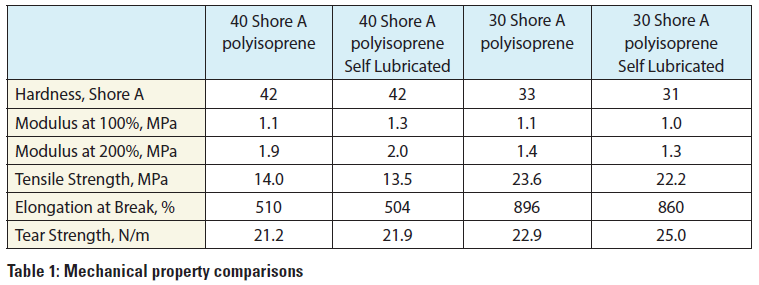
To address this challenge, chemists at Parker Hannifin Corporation’s Medical Systems Division recently developed a family of self-lubricated polyisoprene materials, which have been formulated with a proprietary lubricant system, that show a minimal change of physical and mechanical properties. As shown in Table 1 above, there is minimal reduction of physical properties in the self-lubed versus standard polyisoprene formulation. This family of polyisoprene compounds, which can pass ISO 10993, have a Shore A hardness range from 25 to 50 durometer.
Initial customer feedback from both North American as well as European medical device manufacturer have corroborated Parker MSD’s test results for the above self-lubricated polyisoprene compounds.
With the introduction of this new set of Parker’s self-lubricated polyisoprene materials, medical device manufacturers can expect improved (less) needle drag while retaining the normal functional features (sealability) for polyisoprene septums and injection sites used in drug infusion applications.
Saman Nanayakkara is a Sr. chemist and lab manager at Parker Hannifin Medical Systems Division, responsible for material development and technical service. Shu Peng is an engineering manager for the company, responsible for product design and development.
Article re-posted with permission from Parker Hannifin Life Sciences Division.
Original content can be found on Parker’s Website.
Gallagher Fluid Seals is an official distributor of Parker's products. To speak with a Gallagher engineer, call 1-800-822-4063