Gallagher Fluid Seals is a Parker Distributor is proud to be proud to be one of the few premier distributors to qualify as a “Parker Seal Technology Center (STC)”.
In this three-part article series, we discuss silicone processing options for life science applications. In Part one, we explore the types of silicones, what makes it useful and safe for life sciences, and the chemistry behind it. Part two is devoted to processing. Part three delves into the interplay of design and economics of silicones. In discussing these elements, we hope to provide some thought-provoking ideas about the design and manufacture of medical-grade silicone products.
First, let's talk about the types of silicones that are best for life science applications.
Types of silicones for Life Sciences
Two types of silicones are frequently considered for life science applications: high consistency rubber (HCR) silicone and liquid silicone rubber (LSR).
HCR and LSR are available in a wide range of hardness. The hardness of silicone elastomers is achieved by the addition of silica and other ingredients into the matrix during a process known as compounding. By altering the silicone compound formulations, formulators design silicone compounds from hardness of 10 Shore A to 80 Shore A. (Shore A scale is a typical scale used to characterize hardness of elastomers.) Further, compounders and fabricators formulate silicone compounds to enhance their inherent characteristics or add new features such as colors, low coefficient of friction, ease of processing, adhesion to surfaces, and so on, as needed for the intended application.
What makes silicone useful and safe for Life Science applications?
The reason silicones are so widely used in life sciences is the fact that they have such a wide variety of unique properties:
- Biocompatible with living (human) tissue
- Non-reactive — chemically and biologically inert
- Non-porous
- Low thermal conductivity makes it resistant to heat and thermally stable in extreme heat or cold
- Flexible over wide temperature range
- Once cured, it contains no plasticizers that can leach or be extracted
- Hydrophobic (water resistant) traits inhibit (but not eliminate) microbial growth
Such unique properties of silicones allow the designers to design products from everything from ventilation masks to catheters to SEPTA to molded grommets to medical tubing to extruded profiles.
Properties of silicones that make it useful for Life Science
Siloxane bonds have much greater bond energy compared to C-C bonds, therefore, will not break at temperatures as high as 400°F (200°C) or certain grades up to 575°F (300°C). Silicone polymer molecular structure is highly flexible, resulting in low glass transition temperature (Tg). They are, therefore, flexible and functional at negative or freezing temperatures. The hydrophobic methyl groups in the structure are accountable for its water repellency. Silicone elastomers have unique chemical and physical properties with excellent biocompatibility and biodurability and are ideal for many life-science applications. Other notable characteristics of silicones are excellent resistance to aging, low extractables, and low leachable.
Biocompatibility is one of the desirous characteristics as well as the ability to meet a variety of healthcare and medical industry requirements. Silicones are formulated conveniently to comply with USP Class VI standards and ISO-10993 regulatory requirements, which include:
- Compliant with cleanliness requirements and cGMP requirements
- Sterilizable with steam (autoclave) or gamma irradiation
- Do not contain latex, organic plasticizers or phthalates
The chemistry of silicones
Silicone is a very desired material in the life sciences and other industries. Unlike other synthetic elastomers made of hydrocarbons, Silicone is derived from sand (SiO2), water and natural gas derived methanol. Common organic synthetic elastomer molecules have repeating carbon (C) atoms. The chemistry on the top of the figure below is the chemical structure for synthetic polyisoprene. As can be seen, in the backbone chain, those black elements are carbon-to-carbon (C-C) and there is a carbon-to-carbon (C-C) chain or repeating chain structure, which is the basis for many synthetic elastomers.
On the bottom of the figure is the key differentiating characteristic, which is the silicon (Si) and oxygen (O) substituted chain versus that C-C chain of polyisoprene.
Silicone, also known as polysiloxane, is a chain of siloxane bonds (-Si-O-Si-). Organic units are introduced to the silicone molecules to add various characteristics. With two methyl groups attached to the backbone, polydimethylsiloxane (PDMS) is the most widely used silicone. The siloxane structure and elements used in the backbone of silicone polymer enables us to have unique and desirable properties for life science applications.
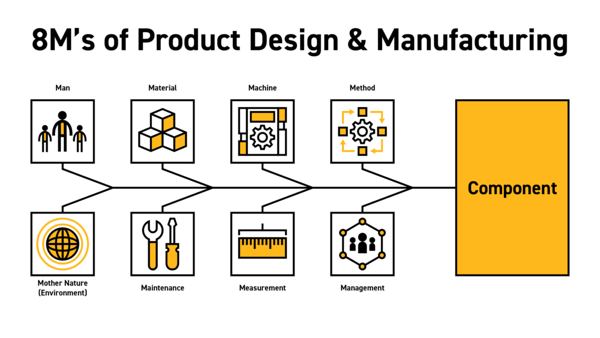
Lastly, let's look at the famous Ishikawa diagram, also known as a cause-and-effect or fishbone diagram. For our purposes, we are using the eight M’s version, which covers man, material, machine, method, management, measurement, maintenance and Mother Nature (environment). This article series will not cover all eight elements of designing and manufacturing a silicone component for life sciences. Instead, we will address two of these: the material and the method of manufacture and their interplay with design and economics. We will cover the balance of these eight M’s in future pieces.
8M's of Product Design & Manufacturing
The original content can be found on Parker's website here.
Gallagher Fluid Seals is a Parker Distributor is proud to be proud to be one of the few premier distributors to qualify as a “Parker Seal Technology Center (STC)”. Contact us today.