The thing with an O-ring is as long as it’s doing its job, you never really even know it’s there. It is only when an O-ring starts to fail and a leak develops that you investigate the problem.
One of the key causes of O-ring cracking is ozonolysis (ozone cracking). Ozone cracking occurs mostly with Nitrile Rubber o-rings (nitrile, buna, buna-N).
What Causes O-ring Cracking
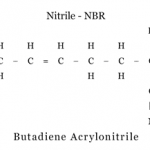
Ozone cracking occurs mostly with O-rings made from nitrile rubber. If you have previous experience with O-rings, you may recognize this material as nitrile, buna, or buna-N. This material is called a polymer, which is Greek for “many units”. Each molecule consists of individual units, which are bonded together into a long chain. In the case of nitrile rubber, the repeating unit, or link, is shown below.
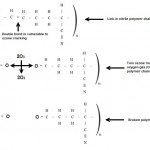
The C, H, and N represent carbon, hydrogen, and nitrogen, while the dashes represent single, double, and triple bonds. Of particular interest is the double bond between the second and third carbon atom. This double bond is a "weak spot" within the molecule. Ozone can "donate" an oxygen atom and break this chain, as shown below.
The two oxygen atoms are connected to the carbon atoms on either side, but they are not connected to each other. Because of this, the polymer chain is literally cut, forming a tiny crack in the O-ring. As more of these polymer chains are cut, the cracks get bigger and bigger, until they can be seen with the naked eye.
How to Prevent Ozone Cracking
Oxygen is present in the air that we breathe, and is necessary for life on Earth. Oxygen atoms typically join up in pairs, forming dioxygen. This makes up the vast majority of oxygen in the atmosphere. Occasionally, oxygen atoms join in groups of three. This creates a substance called ozone.

But in the troposphere, or the air that we breathe, concentrations above 75 parts per billion can cause health problems, according to the EPA (even though their own scientists recommended 60 parts per billion). Even very tiny concentrations such as these can cause ozone cracking in nitrile O-rings.
In general industrial applications, the primary drivers of ozone concentration are ultraviolet light, electrical arcing, and electromagnetic fields (which are the main reasons for higher ozone concentrations in the stratosphere).
Recommendations for storing O-rings
• Keep O-rings away from ultraviolet light. The most common sources are direct sunlight, and fluorescent light bulbs.
• Do not store O-rings within six feet of an electric motor, or other potential sources of electrical arcs.
• Do not store O-rings in a stretched state. O-rings typically need to be stretched for ozone cracking to occur.
Recommendations for installing O-rings
• When using nitrile O-rings on fittings, we recommend installing them into the mating part within 24 hours of installing the O-ring on the fitting. If O-rings must be stored in a stretched state, store them in an airtight bag until ready to use.
• Assemble nitrile O-rings wet with a grease to protect from ozone.
In applications where long-term environmental exposure is inevitable, we recommend using an ozone-resistant material, such as HNBR, EPDM, or fluorocarbon.
This article was contributed by David Mahlbacher, an applications engineer in the Parker O-ring division, and originally seen onthe Parker blog, under the Sealing & Shielding section.