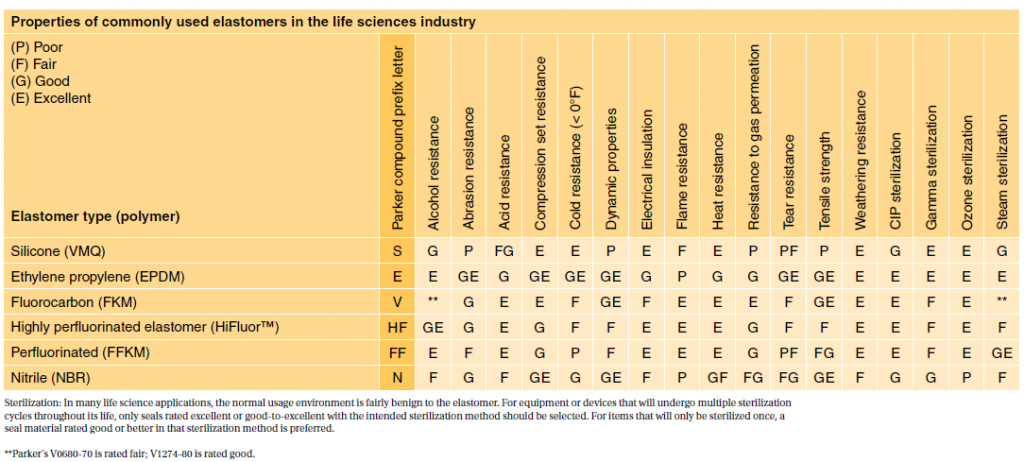
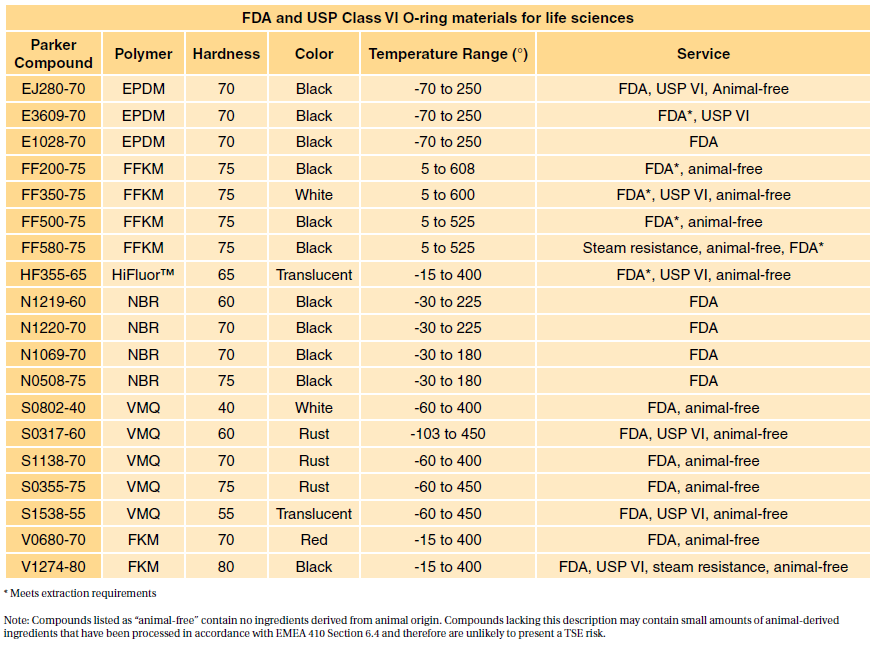
Parker provides a wide range of specialty elastomers to accommodate the various critical sealing challenges presented by the life sciences industry. Selecting a suitable material is critical to patient health. For this reason, the FDA provides a standard (21 CFR177.2600) defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity.
For most patient-contact applications, a material that meets US Pharmacopeia (USP) Class VI and/or ISO 10993/3 will be required. Most applications are fairly benign to elastomers. However, some applications such as implantable devices are extremely complicated. Please contact our engineering department for assistance in selecting materials in these situations.
Features & Benefits
- 7 USP Class VI materials (EPDM, silicone, fluorocarbon, and perfluoroelastomer)
- 24 materials which are compliant to FDA, 21 CFR177.2600
- Specially formulated for long term sealing
- Compounds made without animal-derived ingredients (BSE/TSE concerns)