Introduction to the World of Perfluoroelastomers
The use of elastomers is widespread in our world. Elastomers have many uses including: sealing fluids, for tires, in chemical plants, in semiconductor manufacturing equipment, for dust and moisture seals on cell phones, and seals on aircraft engines. The function of the elastomer and technology involved can vary from something as simple as a barrier to rain water, to seals in automobile engines, to critical sealing applications on the Space Station. Selection of the correct elastomer in an application is very important for successful and long term equipment operation. Although many different elastomers exist in the marketplace, when the highest service performance is needed, in terms of chemical and high temperature resistance, the choice is perfluoroelastomers. 
A perfluoroelastomer can be represented by the letters: FFKM or FFPM (ASTM and ISO designations, respectively). The word itself has two parts, perfluoro (meaning fully fluorinated), and elastomer. Perfluoroelastomers exhibit many properties similar to PTFE (polytetrafluoroethylene), which is also fully fluorinated and considered inert to almost all solvents. PTFE is often referred to as Teflon®, which is a registered trademark of The Chemours Company. PTFE is a plastic, and when compressed, will not recover to its original shape. However, elastomers contain crosslinks, which act as springs to give the material resiliency and the ability to recover after a part has been compressed. This resistance to permanent compression (compression set) gives the material the ability to maintain a seal over time. Finally, while whereas plastics are crystalline, elastomers are amorphous at room temperature; they can be easily compressed and will mold themselves to maintain a seal. Given the chemical structure and performance similarities of FFKMs to PTFE, perfluoroelastomers are sometimes referred to as an elastomeric form of PTFE.
History
Perfluoroelastomers were first developed in the late 1960s, and were trademarked as Kalrez® perfluoroelastomer parts, by The DuPont Company. Many of the early uses for perfluoroelastomer parts were in applications involving high temperatures or aggressive chemicals, including seals used: down-hole in oil wells, for the space program and in chemical processes. Perfluoroelastomers offered improved performance over fluoroelastomers, which were previously the highest performing elastomer parts. Fluoroelastomers are also fluorinated, but they are not fully fluorinated. These products have excellent performance as a material class, but they do not have the chemical and high temperature resistance of a perfluoroelastomer. Viton® is an example of a fluoroelastomer and is a registered trademark of The Chemours Company.
Over time, more and more applications require the high performance of FFKM materials. Aircraft engine manufacturers use perfluoroelastomers in their gas turbine engines to increase flight time between seal repair/replacement. In chemical plants, perfluoroelastomers have been able to increase mean time between repair (MTBR) in mechanical seals in process pumps, resulting in more production and less downtime. FFKM use in the semiconductor industry continues to increase. Semiconductor manufacturing equipment is using higher temperatures and more aggressive gases. Minimizing downtime in this equipment is critical, hence high performance elastomers, such as FFKMs, are used in more and more applications.
Chemistry
The first synthetic elastomer was Neoprene (polychloroprene), which was developed by The DuPont Company in 1930. This material exhibited improved chemical resistance and heat resistance when compared to the elastomer available at that time, natural rubber. After this development, numerous other advancements were made in the emerging field of synthetic elastomers.
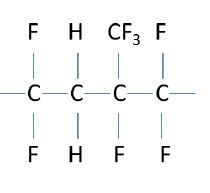
In the 1950s, the DuPont Company created a new synthetic elastomer which had fluorine bonded to the carbon on the backbone, creating a “fluoroelastomer.” This was a major advancement in elastomers, yielding a product that had greatly improved chemical and temperature resistance. An example of a basic fluoroelastomer structure is shown to the right.
Fluorine is the most electronegative element and is extremely reactive, readily bonding with the carbon on the polymer backbone. It is also a relatively large atom which helps to protect the carbon backbone from chemical attack. Unfortunately, a weak point of fluoroelastomers, as depicted in the above structure, is the carbon-hydrogen bonds.
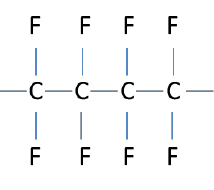
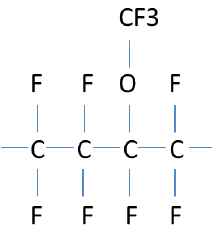
In perfluoroelastomers, the backbone is completely surrounded and protected by fluorine atoms. In this respect FFKMs are similar to PTFE. However the major difference is vinyl ether side groups and crosslinking. The vinyl ether side groups protrude from the backbone, preventing the long chain polymers from lining up, which would result in a more crystalline (plastic) material, such as PTFE. The structure of PTFE can be seen to the right.
Below is an example of a perfluoroelastomer. Note the bulky vinyl ether group, which serves to prevent the long polymer chains from lining up. This material is soft and flexible at room temperature.
The carbon-fluorine bond is also the strongest, most stable bond in organic chemistry; the bond energy is approximately 115 kcal/m. This compares to the carbon-hydrogen bond energy, which is approximately 98 kcal/m. This high bond strength is another reason why perfluoroelastomers are so resistant to chemical and temperature degradation.
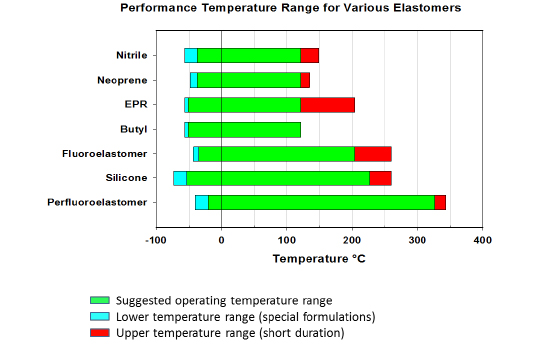
The following chart shows the useful temperature range of some standard elastomers.
Another factor that separates a plastic from an elastomer is its ability to recover to its original shape, or close to it, after compression. This is achieved by crosslinking the polymer chains. Think of the crosslinks as small springs that can stretch when a force is applied that deforms the elastomer. When the force is removed, the crosslinks serve to return the elastomer to its original shape. Different crosslinking systems are available for incorporation into the polymer network.
Note that PTFE and the FFKM are very similar in structure. PTFE is useful up to approximately 200°C. Above that temperature the material softens and readily compresses when a force is applied. For elastomers, the crosslinks help to resist compression and permanent set through a higher temperature range, with some FFKM materials being useful up to approximately 325°C. Unfortunately these crosslinks are not fully fluorinated and can be subject to chemical attack. Different crosslink systems are available for elastomers depending on the intended use. This is one of the reasons different FFKM materials have different temperature ratings. Some may be used up to 225°C while other can be used up to 325°C. Crosslink type can also affect chemical resistance. Typically the high temperature, low compression set FFKM products do not have as broad chemical resistance as FFKM products with a lower upper service temperature and slightly higher compression set. It is necessary to understand the performance subtleties when selecting an elastomer in order to achieve optimum performance results.
About the Author

Russ received a Bachelor of Science in Chemical Engineering from Columbia University in New York and MBA from the University of Delaware.